Air is a mixture of several gases, not a composition. Nitrogen, oxygen, the life-sustaining ingredient for animals and humans, carbon dioxide, water vapor, and trace amounts of other elements make up the atmosphere (argon, neon, etc.). Ozone, helium, and hydrogen are also found in higher levels of the atmosphere. When the wind blows, people can notice the presence of air. Air may now be able to yield a variety of chemically distinct compounds. There’s also dust. Also, there’s water. If you live in a tropical area, you’ll need a lot of water.
If you have wondered if the air is a mixture or not, we have the answers. Read on as we dissect the reasons behind the state of the air.

Air Is A Mixture For The Following Reasons:
- Air is primarily composed of nitrogen (70%) and oxygen (20%), with tiny amounts of carbon dioxide, carbon monoxide, helium, and traces of many other gases.
- There is no chemical reaction between the components, and there are no chemical links between them. Therefore it is a mixture. Each molecule bounces around on its own, unaffected by the others. Collisions rarely result in reactions.
- To the extent that other molecules react chemically with air, each air component reacts individually with each molecule based on temperature and partial pressure.
- Air can provide oxygen for life and oxidation, although partial pressure is crucial in this case. Adding or subtracting oxygen, diluting the air with other gases (e.g., more nitrogen, carbon dioxide), or changing the total pressure can change the partial pressure.
What Does Air Consist Of?
The majority of the gases consist of Nitrogen (N2): 78%, Oxygen (O2): 20%, Noble gasses: 1%, Carbon dioxide (CO2): 0.03%, Water vapor (H2O): 0.97%.
The amount of water in the air fluctuates dramatically. When there is a lot of water in the air, other elements are present in smaller proportions. The quantity of water in the air can increase by up to 4%. The smallest amount of water in the atmosphere is 0.5%. Present gases get closer together by water, allowing them to take up enough space.
The air is usually very ‘dry’ when the amount of water is exceedingly low. A dry air volume of 22,4 dm3 weighs 28,96 grams. Humidity refers to the presence of significant amounts of water in the air. Air that is humidified is lighter (less dense) than dry air.
Other components, in addition to those mentioned previously, are found in the air. The percentages of these elements, however, are pretty low. Aerosols are a type of particle that We can see in the air. These are just dust particles blasted off the Earth’s surface by the wind or emitted during volcanic activity. Ashes and dirt particles are released into the air during the burning process.
The composition of the air also changes as one rises in altitude. Over 90 kilometers above the Earth’s surface, oxygen molecules disintegrate, leaving only oxygen atoms. Nitrogen molecules decay over a hundred kilometers above the Earth’s surface. At this altitude, the air does not have the same composition as it does at ground level. There is a very distinct difference in the atmosphere.

Gas (Air): What it is
We can find every chemical element in a variety of states (phases). The gaseous phase is one of these stages. A component can be solid or liquid in addition to being gaseous. When water is reliable, it is called ice; when it is fluid, it is termed wet; and when it is gaseous, it is known as gas or steam.
When temperatures rise, the molecules of a critical component expand, making the substance gaseous and less visible. Air becomes invisible as a result of this stage transition.
When temperatures drop, air molecules clump together more efficiently, and a substance solidifies. For each substance, the temperature at which each phase change occurs varies.
Where Can We Find Air?
Air can be found everywhere on the planet except where there is water, we can say. It can even be found in the Earth’s surface layer, in the soil. Air is located on the Earth’s surface and in an air layer known as the atmosphere. Depending on temperature and height, the atmosphere can be separated into distinct layers. Straight borders do not adequately distinguish these lines; they constantly overlap one another.
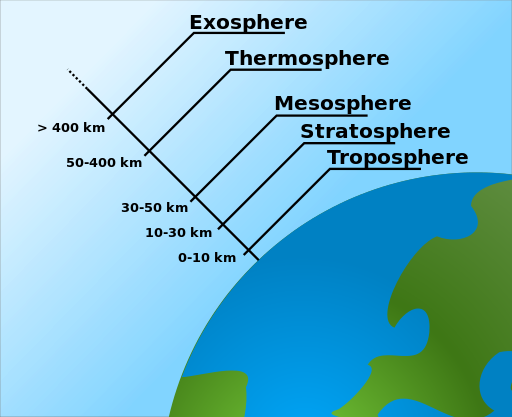
The Troposphere
The troposphere refers to the first layer of air, which is closest to the Earth. This stratum has a height of 11 kilometers. Temperatures drop six or seven degrees each kilometer as you ascend into our troposphere. Thus the weather on Earth is mainly stable by conditions in the troposphere.
The tropopause is the upper stratum of the troposphere. This layer is eight to ten kilometers above the surface of the planet near the Antarctic. The tropopause layer is observed about seventeen to eighteen kilometers above the Earth’s surface at the equator.
The Stratosphere
The stratosphere is the second part of air above the troposphere. In the bottom part of this layer, temperatures stop dropping. The temperature has dropped to roughly -55 degrees Celsius. Temperatures in the upper stratosphere are rising to zero degrees Celsius about 47 kilometers above the Earth’s surface.
Between twenty and forty kilometers above the Earth’s surface, solar energy creates ozone (O3) from oxygen (O2) in the stratosphere. The ozone-sphere is there to this region of the stratosphere as a result of this reaction. The stratosphere is the uppermost part of the stratosphere.
The Thermosphere
The thermosphere, the fourth layer of air, can be found at the height of ninety kilometers above the planet. Temperatures climb dramatically in this layer, with the most significant temperature reaching over 1000 degrees Celsius. Because the air density in this layer is so low, the strengths between molecules are almost non-existent.
The Exosphere
The exosphere, the lowest layer of the thermosphere, allows the lightest molecules to escape. Because it disappears into space, the exosphere lacks a distinct boundary.
The Hemisphere
Because the air composition is consistent primarily, the lowest ninety kilometers of the atmosphere are often referred to as the hemisphere.
What Types Of Air Are There?
When a massive amount of air has the same wetness and temperature, it is classified as one type of air. The air type must encompass a thousand-kilometer flat area. Its type’s height might range from a few hundred meters to covering the whole troposphere.
When a gas mass circulates for three to nine days in a region totally above either land or water and where the wind does not blow, an gas type forms, the gas mass obtains its unique qualities in this location. These habitats might be deserts or savannas above ground.
As soon as the gas leaves the location, its unique characteristics begin to weaken and eventually vanish. Air types that obtained their specific properties above sea level are substantially more humid than those that received their respective features above land level. The air types that form above seas are also marine gas types. Continental air types are gas types that are shaping above land.
We can differentiate four basic gas types, all of which can be classified into marine and continental categories:
- Air from the equator. Temperatures also range from 25 to 30 degrees Celsius, with high moisture content.
- Air from the tropics. Though moisture content of tropical marine air is high, and the temperature is around 25 degrees Celsius. Tropical air from the continent also has low moisture content and more than 50 degrees Celsius.
- The gas is also polar. The marine polar air is always humid, hot in the winter, and cold in the summer. In the winter, continental polar gas is arid and frigid. Temperatures as low as -50 degrees Celsius are possible. This type of air is warm in the summer yet still relatively dry.
- The air is arctic.
Frequently googled questions on Air, a mixture.
Therefore, we present you with the most googled questions on air.
Air, a homogeneous mixture
A homogeneous mixture is one in which all particles have the same, or nearly equal, composition. In other words, the constituent particle concentration remains constant over the total volume under consideration. Saltwater is an example of a homogeneous mixture because when sodium chloride particles are getting into water, they dissolve, and the concentration of the solution remains constant at any point in the solution.
Is air a pure mixture?
Air is a mixture of many elements in just the proper proportions to sustain human existence on Earth. Hence it is not a pure material. A pure material has the same chemical makeup throughout its entire life cycle. Ir is a combination of nitrogen and oxygen, accounting for 78% of the total (20 percent ). However, because air contains various gases in varying proportions, we refer to it as a mixture rather than a pure entity.