Substances combine chemically to create new substances. The new substances have properties completely different from the initial substances. The initial substances that undergo the reaction are called reactants. The reaction between the reactants creates the substances called the products.
In other words, a chemical reaction is in which we break the bonds within the reactant molecules, to form new bonds within the product molecules in order to create a new substance.
Chemical reactions occur through changes in energy within the reactants. The products henceforth, have physical and chemical properties completely different from that of the reactants. Therefore, chemical reactions are thermodynamic in nature.
Due to the unimaginable number of chemical reactions happening in our surroundings, a nomenclature was created to simplify the expression of a chemical reaction in the form of a chemical equation. A chemical equation is a mathematical statement that symbolizes the product formation from reactants. It also states certain conditions under which the reaction has been conducted.
The reactants are written on the left-hand side whereas products formed during the process are presented on the right-hand side are connected by one-headed or two-headed arrows. For example, a reaction-
A + B → C + D
Here, A and B react to form the products C and D. In an actual chemical equation, we denote reactants by their chemical formula. In order to assure the correctness of the law of conservation of mass, we should always balance a chemical equation and make sure that the number of atoms on both sides must be equal. Henceforth, we obtain the balanced equation.

Examples of Chemical Reactions
Here are some common examples of chemical reactions that we encounter in daily life:
- Photosynthesis
- Aerobic cellular respiration
- Anaerobic respiration (including fermentation)
- Rust formation on Iron
- Electrochemistry (including chemical batteries)
- Digestion
- Soap and detergent reactions
- Cooking
- Fireworks
- Rotting of food
- Electroplating of metals
- Disinfecting surfaces and contact lenses

Types of Reactions
- COMBINATION REACTIONS
- In such a reaction, two or more compounds combine to form one compound with properties completely different from that of the parent compounds.
- A + B → AB
- DECOMPOSITION REACTIONS
- Such a reaction is the opposite of a combination reaction, where a complex molecule breaks down to form many simpler ones.
- AB → A + B
- PRECIPITATION REACTIONS
- In such a reaction, two solutions of soluble salts are mixed resulting in the formation of an insoluble solid (precipitate).
- A + B (soluble salt) → Precipitate + C (soluble salt)
- NEUTRALISATION REACTIONS
- Here, an acid and a base react with each other. Generally, the reaction of this kind produces salt and water.
- Acid + Base → Salt + Water
- COMBUSTION REACTIONS
- During combustion, Oxygen combines with any other compound to release carbon dioxide and water. These reactions are exothermic, which signifies that they release heat during the process.
- A + O₂ → H₂O + CO₂
- DISPLACEMENT REACTIONS
- One element shifts place with another element in the reactant compounds, during such a reaction.
- A + BC → AC + B

What Is Combination Reaction?
A combination reaction is defined as a chemical reaction where two or more compounds react to form new products. A combination reaction is also referred to as a synthesis reaction.
The general formula of a combination reaction is denoted as:
A + B → AB
Where A and B are the reactants that combine to form the product AB. AB has properties completely different from its parent compounds.
Examples of Combination Reaction
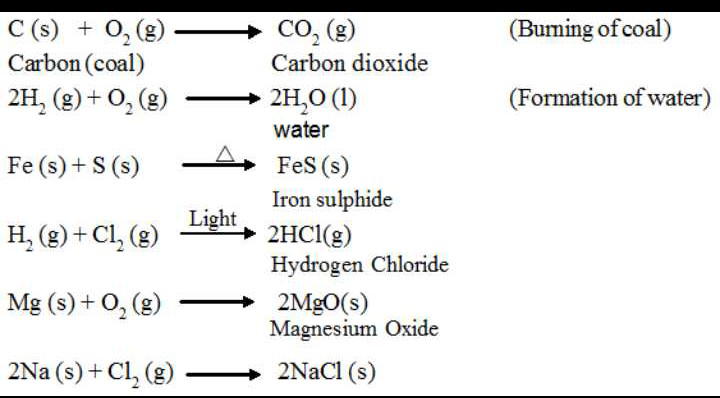
A simple example of a combination reaction is when two elements combine to form a different compound. Just like when solid Sodium metal reacts with chlorine gas to produce solid Sodium Chloride.
2Na(s) + Cl₂ (g) → 2NaCl(s)
Another kind of combination reaction that occurs frequently is the reaction of an element with oxygen to form an oxide. Consequently, metals and non-metals both react readily with Oxygen under the most viable conditions. For instance, Magnesium reacts rapidly and dramatically when ignited, combining with Oxygen in the air to produce a fine powder of magnesium oxide. This is often used to give signals.
2Mg(s) + O₂ (g) → 2MgO(s)
In another case, Sulfur reacts with oxygen to form sulfur dioxide. This is also an example of a combination reaction.
S(s) + O₂(g) → SO₂(g)
When non-metals react with one another, the product is a molecular compound. Often, the non-metal reactants can combine in different ratios and produce different products. Sulfur can also combine with oxygen to form sulfur trioxide.
2S(s) + 3O₂(g) → 2SO₃(g)
Transition metals can adopt multiple positive charges within their ionic compounds. Therefore, most transition metals can form different products in a combination reaction. For instance, the reaction where Iron reacts with Oxygen to produce both Iron (II) oxide and iron (III) oxide:
2Fe(s) + O₂(g) → 2FeO(s)
4Fe(s) + 3O₂(g) → 2Fe₂O₃(s)
Most Combination Reaction are Exothermic in nature. Why?
Combination reactions are usually exothermic in nature. Combination reactions usually involve the creation of new bonds and this process releases a large amount of heat energy in the environment.
Read Also: Post-Apocalyptic Literature: Definition & Books
Despite there being multiple exceptions to this, usually, all combination reactions are exothermic in nature.
An exception is the high-temperature combination of nitrogen and oxygen to form nitric oxide (NO).
N₂(g) + O₂(g) → 2NO(g)