A PH calculator is a tool that users can utilize to find out the pH value of any solution on the internet. If you use a pH calculator you can save time in calculations and get accurate results at the same time. Thus, pH calculators are efficient not only in precision but also are free and can increase the quality of your work with correct measurements.
If you have ever struggled with calculations of acidity and basicity of a chemical then a pH calculator may be the solution. Keep reading to know more about the use of the pH scale and how to use the pH calculators available on the internet.
How to Use a pH Calculator?
These are the steps that you can follow in order to calculate the pH of a chemical solution using a pH calculator. Firstly, you have to type in the name of the chemical solution in the pH calculator. The pH calculator will also provide you with a field to enter the concentration value of the solution. Thus enter the concentration of the solution whose pH you want to measure.
Next, you have to click on the calculate button. Thus, the application will calculate and display the pH value of the solution according to the details that you have entered. Finally, the pH calculator will make the final results visible to you in a new window.
What is Meant by pH Measurement?
In chemistry, the term pH refers to the hydrogen potential of a solution. So it is very important to understand what the term hydrogen potential means. If we perform electrolysis of water, the amount of hydrogen that is released will be the hydrogen potential of water.
Similarly, for other chemical solutions, the amount of hydrogen that electrolysis releases gives the hydrogen potential of that solution. The pH of a solution thus measures the acidity or alkalinity of the solution. It measures the number of hydrogen ions present in a solution. Thus, the hydrogen ion concentration of a solution gives the acidity or the alkalinity of the solution.
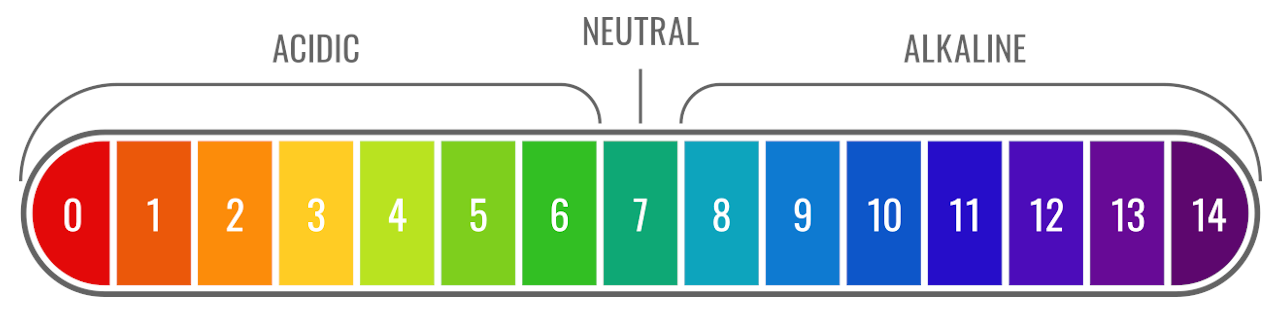
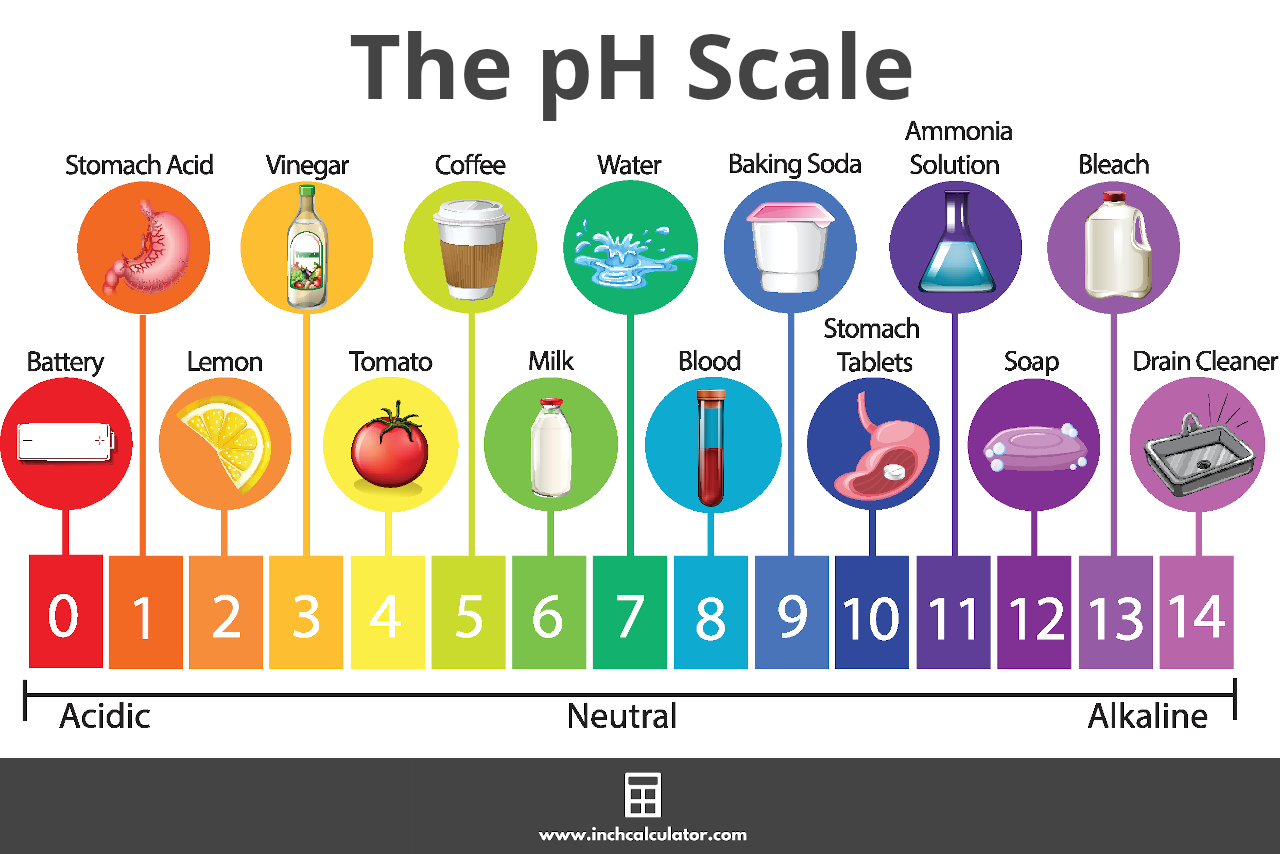
On the pH scale, we have values ranging from 0 to 14. Therefore, based on the number on the pH scale we can determine the number of h+ ions in a solution and hence its acidity or basicity. If the value on the pH scale is greater than 7 then the solution is acidic.
This is true for all aqueous solutions with a temperature of 25 degrees celsius which have a pH of more than 7. However, if a solution has a pH of less than 7, it is basic or alkaline in nature. Let us take into consideration a chemical solution of acetic acid. Acetic acid has a molecular weight of 5.
Thus if we calculate pH based on molarity, the pH value of a 5 molar solution of acetic acid is 2.03. Since the value is much lesser than 7, acetic acid is a strong acid with high acidity.
Ph calculator from h
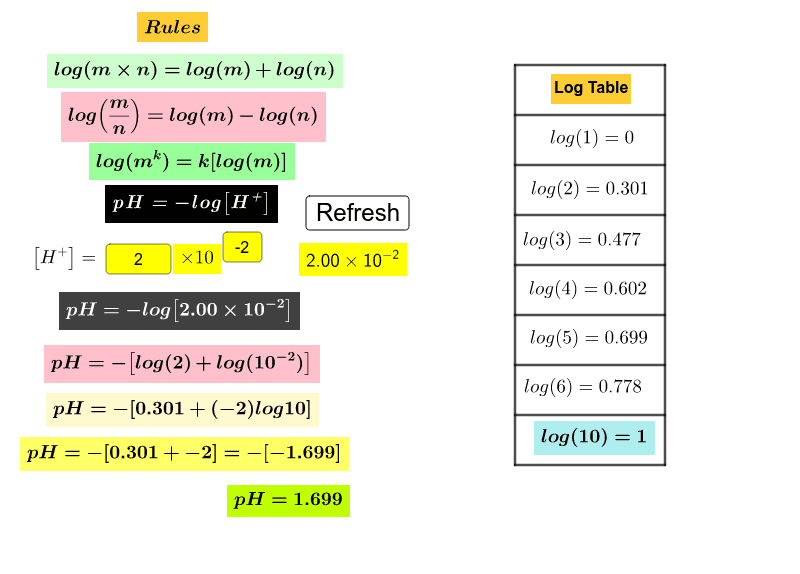
There are many ways to determine the pH of a solution. However, the most common method that we use to calculate the pH of a solution is by taking the concentration of hydrogen ions into consideration. Thus the pH of a solution is the number of hydrogen ions present in the solution in comparison to water.
It is a measure of the relative hydrogen ion concentration between water and a solution. Thus, this factor determines whether a solution is acidic or basic. From the hydrogen ion concentration, we can determine the pH of a solution by taking its negative logarithm. Acids and acidic solutions will have an H+ concentration higher than that of water.
Bases or basic solutions have an H+ concentration which is lower than that of water. Thus, acids have a hydrogen ion concentration greater than 1 × 10^{-7} M. Bases have a hydrogen ion concentration less than 1 × 10^{-7} M.
Thus, both blood and the protoplasm or cytosol have a pH of 7 to 8. The inside of the cells in the human body is mostly in an aqueous medium. If we are calculating the pH of a water-based or aqueous solution, it increases with the addition of bases and decreases with the addition of acids. Thus, aqueous solutions gain and lose hydrogen ion concentration depending on whether we add acids or bases to them.
What are acids and bases?

Since we have spoken in such detail about the pH scale and the pH of various solutions it is also important to understand the concepts of acids and bases. What are acids and bases? We can also answer this question with the concentration of hydrogen ions in a particular solution. If we take the negative logarithm of the hydrogen ion concentration of pure water, we will get a value of 7.0.
The hydrogen ion concentration of pure water which we obtain by electrolysis is 1 × 10^{-7} M. We know the value of 7 on the pH scale as the “neutral pH”. Thus we can define an acid as a solution that has a value of less than 7 on the pH scale. We can define a base as a solution that has a value of more than 7 on the pH scale. The cells inside the human body usually have a pH of 7 or 8 which is an acidically neutral environment.
According to the context, an acid is a substance that will increase the concentration of H+ in a solution. Acids do this by dissociating one of their own hydrogen ions. Thus, on the other hand, a base will decrease the concentration of H+ ions in the solution and raise the pH. Bases can do this by removing the hydrogen ions that were already present in the solution with hydroxide ions. It can also use other ions or molecules to remove hydrogen ions from a solution.
Ph calculator dissociation
We can also calculate the pH of a solution from its degree of dissociation. If an acid readily dissociates in solution to give a large amount of H+ ions, it is a strong acid. Thus we can determine the strength of acid by how easily it yields hydrogen ions in solution. For example, when hydrochloric acid or HCl is dissolved in water, it completely dissociates into hydrogen and chloride ions.
Thus, we consider it to be a strong acid. On the other hand, tomato juice and vinegar do not dissociate completely when we dissolve them in water. Some of the molecules break into hydrogen ions and the other respective anions, but most of them do not dissociate. Thus, tomato juice and vinegar are weak acids.
Similarly strong bases are the bases that dissociate completely in water and release basic ions that absorb the H+ ions. For example, when we dissolve NaOH in water, it completely breaks down to yield a large number of hydroxide ions. The hydroxide ions remove most of the H+ ions present in the solution and thus increase the pH.
Ph calculator from base
In physical chemistry, we use the pH scale to measure the acidity and the basicity or the alkalinity of solutions. pH is actually the negative logarithm of hydrogen ion concentration of solutions. Thus it is the negative of the exponent to which we have to raise a base to get a certain number.
If there is a change in the pH value of a solution, it will correspond to a tenfold change in the concentration of hydrogen ions. This is because the pH scale is based on the amount of H+ ion concentration. Chemists have marked the pH scale with values from 0 to 14. Thus, most solutions will have a pH value which is between 0 to 14.
However, it is really interesting for us to know that some solutions also have a pH that is beyond this range. It is possible for us to get a pH value of a solution that is more than 14 or less than 0. Thus, we consider anything less than 0 to be just acidic and anything more than 14 to be just basic or alkaline.
Ph calculator online
We can find many pH calculators on the internet that can calculate the pH of biological cells. Thus, we can use these tools to get to know about the biochemistry of our own bodies. Inside the human body, most cells have an aqueous environment with a pH very close to neutral. Thus, the pH of blood is 7.4 which is slightly alkaline, and the pH of human cells is 6.8 which is slightly acidic.
The human body maintains this pH inside its cells to provide a favorable environment for the various reactions to take place. Thus, biologists consider it very unfavorable for the pH of live cells to be more or less than the normal range. If the pH of our cells becomes more acidic or more basic, it can hamper bodily functions. Even though the stomach is highly acidic to enable the digestion of food, the stomach cells are quite basic.
The stomach has a pH of about 1 or 2, while the internal cells of the gut have a regular neutral environment. It is very interesting to understand how the stomach manages to maintain this unlikely scenario. The answer is cells that have a lifespan of just a few days! The stomach regularly throws away cells that come in contact with its acidic exterior.
The cells of the gut lining that regularly come in direct contact with the acid in the stomach and the food have a very low lifespan. Thus they constantly die and the stomach regularly replaces them. Hence about every seven to ten days our body completely replaces the inner lining of the stomach.
Ph calculator Buffer

For most organisms including humans, the pH needs to exist in a narrow range. Thus during the lifetime of a human, they must maintain a pH of 7.4 in their blood. If there is a significant increase in acidity or alkalinity in human blood it can lead to many diseases and unfavorable conditions.
In other words, irrespective of the substances that enter or leave the human bloodstream, the blood must maintain a certain range of pH to be healthy. In order to achieve this task, solutions known as buffers exist. Buffers are solutions that can resist changes in their H+ concentration. The main function of a buffer is to maintain the pH in biological or chemical systems.
Thus, in such biological cases, buffers maintain the stable hydrogen ion concentration. If hydrogen ions accumulate in the water a buffer absorbs some of them and thus brings the pH back to normal. On the other hand, if there is a deficiency of hydrogen ions in the water a buffer donates some of its H+ ions to regulate the pH. Usually, buffers consist of a pair of acids and bases. In this pair, the acid and the base differ by the presence or the absence of a proton. Thus they are also called a conjugate acid-base pair.
Ph calculator bicarbonate
Two important buffers that maintain the pH of human blood are carbonic acid (H2CO3) and the bicarbonate ion (HCO3-).
In the human body, carbon dioxide is generated in the lungs and it is converted back to water and carbon dioxide and released as waste. Thus, we have to first understand how carbon dioxide travels in the blood. In human blood, the carbon dioxide dissolves into carbonic acid. Thus it combines with water in the bloodstream and moves back and forth from the lungs and the different muscles of the body.
We can represent this in the form of an equation which is (H+)+ HCO3- > H2CO3. If there are too many H+ ions in the blood, that is if the pH of the blood is low, this equation will shift to the right. Thus, the bicarbonate ions will absorb the hydrogen ions in order to form carbonic acid. On the other hand, if the hydrogen ion concentration falls below a critical point, that is if the pH of the blood increases, the equation will shift to the left.
Thus, in this case, the carbonic acid will convert back to bicarbonates and thus donate H+ ions to the solution. If the pH of the blood fluctuates too much, it can be fatal for the body. Thus, buffers play a very important role in maintaining the pH and thus ensuring the survival of living creatures.
Frequently Asked Questions About pH Calculator
How do you solve for pH on a calculator?
The procedure to use the pH calculator is quite simple. First, you have to enter the name of the solution whose pH you want to calculate. You also have to enter the concentration of the solution in molarity. The application will provide fields in which you have to enter this information. Finally, you have to click on the calculate button to get the results. Thus, the pH calculated will display the calculated pH value in a new window.
What are pH and its formula?
The pH and its negative logarithm are Sorensen’s definitions of hydrogen concentration and its pH. The pH of an aqueous solution can be calculated based on the hydronium ion concentration using the equation pH=−log[H3O+].
How do you calculate H+ from pH?
In the following equation, pH = −log [H+], where [H+] denotes the molar hydrogen ion concentration. In order to calculate pH, we must take the common (base 10) logarithm of hydrogen ion concentration.
What is a pH of 10?
The pH scale ranges from 0 to 14. A pH of 7 is neutral and a pH of less than 7 is acidic. A pH greater than 7 is basic.