This article explains the formula for sodium nitrite. It is an inorganic compound that is an extremely unstable alkali metal nitride. To make it, atomic beams of nitrogen and sodium are mixed and deposited at low temperatures on a sapphire substrate. As a result, it decomposes quickly into its component elements. The chemical formula for sodium nitrate is Na3N. At ambient temperature, the bandgap is that of a semiconductor, and over 90% of the atoms are ionic. A basic lattice comprised of Na octahedra serves as an anti-ReO structure. Between Na and N, the bond length is 236.6 pm. The structural conformation of a material can be determined using X-ray diffraction.
The color of the compound varies depending on how it is made, from reddish-brown to dark blue. There is no melting point for this substance.
If you wanted to know more about Sodium Nitrite, you are welcome here. Read on as we shed ample light on this topic.

Sodium Nitrite Chemical Formula
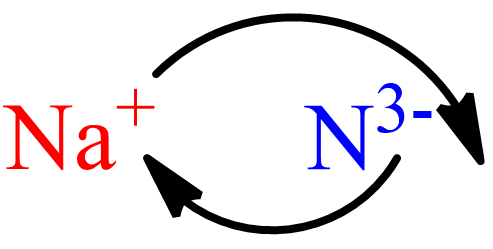
The Sodium nitrite is an inorganic chemical since it lacks carbon-hydrogen atoms. Sodium nitride comprises very unstable alkali metal nitride, according to its chemical formula. Sodium belongs to group 1 of the periodic table. Electrons also generate in this scenario by losing 1+ions, such as Na+, resulting in single free electrons.
The element nitrogen is also in Group 5 of the periodic table.
When three electrons are there, a nitrogen ion is also there, resulting in N3-ions. They are made by combining nitrogen and sodium atoms that also place on sapphire at low temperatures. The molecular mass of sodium nitrite is 82.976 g. The ionic component also form by balancing the constituent ions. As a result, the ionic components also assemble by balancing three sodium ions with one nitride ion.
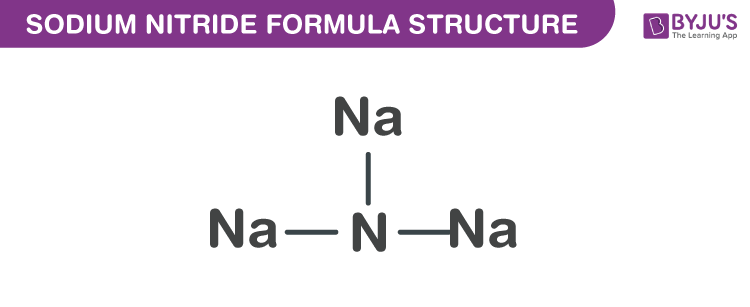
The chemical formula for sodium nitrite is Na3N. It’s just as brittle as sodium nitrite. Sodium and nitrogen are the two elements that make up sodium nitride and easily dissolve in each other.
So the formula of Sodium Nitrate is 2 Na3N → 6 Na + N2.
Sodium Nitrite molecule formula calculation
- Metals preserve their names and are the first to be named and written.
- The nonmetal name retains its primary name now that the ending is -ide.
Ionic compounds are usually made up of a metal (Na), which belongs to Group 1A. It’s got a +1 charge on it. Na+ represents metal ions. The nonmetal analog of nitride is nitrogen. The elements in Group 5A have the ability to produce -3 ions. The nonmetallic ion is N3-. Ionic compounds are formed when the charge of an ion is transferred to the counterion’s subscript. In the formula unit or molecular formula, it will appear as Na3N.
Sodium Nitrite Structure
Um, nitride. A comparable cation also can be there in lithium nitride and potassium nitrite.
The formula for sodium nitride shows that it has an Anti-ReO structure. Sodium nitrite, which is also in Na Octahedra, similarly has a simple lattice structure. The length of the N-Na bond is approximately 236.6 pm. The structural formation of sodium nitrite also is recorded via X-ray diffraction. With sodium amide and sodium amide, it shares the same anion as sodium amide.

Sodium Nitrite Characteristics
Due to intrinsic features, sodium nitrite can be reddish-brown or dark blue in color, depending on the synthesis of the component. When kept at room temperature for several weeks, it displays no indications of breakdown. The molecule has no melting point since it decomposes back into its elemental forms about 360 K, as proven by mass spectrometry. The compound’s estimated formation enthalpy is +64 kJ/mol.
Sodium Nitrite Overview
At room temperature, sodium nitrate has a bandgap equivalent to that of semiconductors. The color of sodium nitride ranges from dark blue-red to reddish-brown. Sodium nitrides come in a variety of hues depending on how they are made. There is no boiling point for sodium nitrite. Sodium and nitrogen ionic compounds have extremely high melting and boiling points. Electrostatic attraction attracts the oppositely charged ions to each other.
With an enthalpy rate of around +64 kJ/mol, sodium nitrite can be produced. Sodium nitrates can be dissolved in water. The temperature at which sodium nitride decomposes is 86.85°C (360°K).

Sodium Nitride Synthesis
we can make sodium nitrite via thermal breakdown of NaNH2 or by the direct approach. When sodium forms in nitrogen and nitrogen are added as sodium ions, a cavity is generated, and the sodium ions reorganize and accommodate close nitrogen. The conduction band of s electrons is formed as sodium ions migrate more relative to nitrogen. An energy bandgap exists between them, resulting in a metallic compound that is analogous to a semiconductor or insulator.
Read Also: Ecological Validity: Definition and Examples
Dieter Fischer and Martin Jansen and Grigori Vajenine have successfully synthesized sodium nitride utilizing the latter approach. The first method involves individually introducing suitable Na and N2 ratios in the gas phase and depositing them in a vacuum chamber on a cooled substrate, which is then heated to room temperature (298 K) to crystallize. On a metal surface, elemental sodium is reacted with plasma-activated nitrogen in the second process. we can aid this synthesis further by adding liquid Na-K alloy to the complex, then removing the excess liquid and washing it with new alloy. A centrifuge is then used to separate the solids from the liquid.
Sodium Nitride Conclusion
The Sodium nitrite is an inorganic chemical, meaning it is devoid of carbon-hydrogen bonds. Sodium nitrite is an extremely unstable alkali metal. They can easily break down into sodium and nitrogen ions as a result. Potassium nitride and lithium nitride both have the same cation as lithium nitride. However, they are both alkali metals. we can make sodium nitrite (Na3N) through the thermal breakdown of NaNH2 or by a direct reaction in either instance. They do a lot of food preservation.





