One of the fascinating substances to learn about is ammonia or NH3. It has a pleasant odor and emits a colorless, fire-resistant gas. One nitrogen atom and three hydrogen atoms make up this compound. Ammonia is a polar molecule because its electron sharing is asymmetric. It has a trigonal bipyramidal molecular structure and is widely utilized as a fertilizer, making it one of the most prevalent components in agriculture. Ammonia is also there in insecticides, building colors, and other chemicals.
To figure out if this molecule is a base or an acid, you must first determine whether it is a base or an acid. The pH of a molecule is commonly used to decide whether or not it is a basic or an acid. Because ammonia differs from other compounds, a one-word answer to this issue would be inadequate. Continue reading to learn whether NH3 is an acid or a basic.

Is Ammonia An Acid Or A Base?
NH3, often known as ammonia, is a pungent-smelling gas compound consisting of one nitrogen atom and three hydrogen atoms. Ammonia is lighter than air and has a low boiling temperature of -33 degrees Celsius. Students frequently debate whether NH3 is an acid or a basic. In this article, you’ll learn everything there is to know about the acidity and basicity of NH3.
Is NH3 a basic or an acid? NH3 is a weak base with a pH of 11 (in typical settings), but it is also amphoteric, meaning it can function as both an acid and a base in different situations. Under ideal conditions, NH3 acts as a weak base, accepting H+ and forming the conjugate acid NH4+; but, under unfavorable conditions, NH3 acts like an overly weak acid, giving away H+ ions and creating the conjugate Base NH2-.
However, following Lewis’s theory of acids and bases, NH3 is considered a Lewis base due to the presence of a lone pair of electrons.
Ammonia: What it is
Ammonia (NH3) is a colorless, fire-resistant gas that is lighter than air. Due to its production by bacterial breakdown of urea has a powerful foul odor and is recognized as a pungent-smelling gas. Ammonia is a very hazardous gas that can cause lung damage or even death if inhaled in large amounts.
Ammonia is widely utilized in industry to make fertilizers, disinfection chemicals, refrigerants, and various other nitrogen-based organic and inorganic compounds.
The chemical formula for ammonia is NH3, and it has a trigonal pyramidal shape with a nitrogen atom at the top and three hydrogen atoms at the bottom. Nitrogen contributes to a single electron pair.
NH3 has a molar mass of 17.03g and a bond angle of 107.5 degrees, which is somewhat less than that of tetrahedral NH3 (109.5 degrees). Because the lone pair adds repulsion to bonds, the angle is slightly less than the tetrahedral.
Is NH3 Acidic Or Basic?
As previously stated, NH3 is a weak base with a pH of 11, but it is amphoteric in nature, meaning it can function as both an acid and a floor under different conditions.
When NH3 works as a base, it devotes its lone pair to a proton H+ and forms the conjugate acid NH4+, but when it acts as an acid, it can release the H+ ion and form the conjugate base NH2-.
(Acting as a Lewis Base) NH3 + H+ → NH4+
(Acting as a Lewis Acid) NH3 → NH2- + H+
The reason behind NH3 acting as a Lewis Base
According to Lewis, any chemical substance that can devote lone pairs to other chemical species can operate as a Lewis base. We know that in NH3, Nitrogen(N) has 5 electrons in its valence shell with the configuration (1s2, 2s2, sp3), but Hydrogen only has one electron with the structure (1s2, 2s2, sp3) (1s1).
Because NH3 is sp3 hybridized, it shares three (sp3) hybridized electrons to form bonds with the 1s electrons of all three hydrogen atoms, leaving one (sp3) electron pair on N unpaired.
It means that one lone pair of electrons is left on the Nitrogen atom, which can contribute to a proton in an appropriate media so that NH3 can behave as a Lewis base.
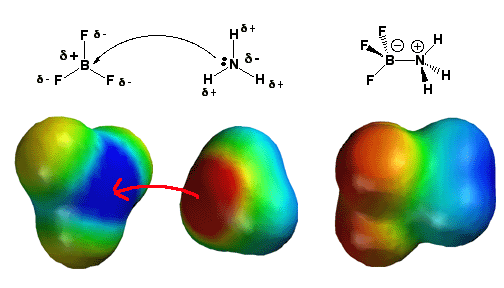
Description:
The BF3 molecule is present on the left. There is one 2p-orbital that is not hybridizing and vacant with three bonds to fluorine (sp2 hybridization) and no lone pairs. To give the boron atom an octet of electrons requires a lone pair of electrons. Fluorine is also extremely electronegative, causing the boron atom to lose electron density. The electrostatic potential model on the upper left depicts this, with fluorine atoms colored yellow-orange (partial negative charge) and boron colored blue (partial positive control).
For all of these reasons, the molecule BF3 is an excellent electron acceptor and a Lewis acid. In the center, ammonia (NH3) is also there. The nitrogen atom possesses a single pair of electrons, and because nitrogen is more electronegative than Hydrogen, it has a partial negative charge (red color). When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3’s vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3). The boron half of the molecule (red hue) has a negative charge, whereas the ammonia half has a positive charge (blue color).
Chemical Properties of NH3 (Ammonia)
According to the VSEPR theory, ammonia has a trigonal pyramidal chemical structure with bond angles of 107.5 degrees. After exchanging three electrons to establish bonds with three hydrogen atoms, nitrogen is there with one lone pair of electrons.
This trigonal pyramidal structure provides a dipole force to the molecule, making it polar. It has the ability to create hydrogen bonds in water because of the presence of a lone pair.
Amphoteric Nature
As previously stated, NH3 is a weak basic that forms salts when it combines with acids. Although NH3 is a soft base, it can also behave as a weak acid and react with bases in specific settings. It can shed H+ ions and generate Amides (NH2-). When Lithium reacts with NH3 to generate Lithium Amide, this is an example of such a reaction.
Li + NH3 → LiNH2 + H2
Redox Reaction
Under some conditions, NH3 also completes self-dissociation and causes a redox reaction. The reaction in which NH3 creates its conjugate acid and conjugate base is available below.
NH3 → NH4+ + NH2-
Combustion
Exothermic combustion produces nitrogen gas and water vapor from NH3. The NH3 combustion reaction is available below.
(The enthalpy change of this reaction is 1267.20 kJ/mol)
Even though Nitrogen oxides are unstable in comparison to N2, we can still make Nitrogen oxides with the help of various catalysts. This is an example of a reaction like this:
NH3 + O2 = NO + H2O
Because of the high heat of vaporization and ignition temperature, NH3 must always be burned with the presence of a catalyst. One of the many catalysts used in the NH3 combustion reaction is platinum gauze.
Acting as Base in water
Because of its polar nature and propensity to create hydrogen bonds in water, NH3 quickly connects with water when placed in it. It aids in the breakdown of H2O molecules into (Hydrogen ions) H+ and (Hydroxyl ions) OH- ions and the formation of bonds with H+ ions.
When NH3 reacts with H+ ions, it produces NH4+ and leaves OH- ions in the solution. Because the concentration of OH- ions rise with the rise in pH, it imparts basicity to the solution. The formed ammonium ion (NH4+) also keeps breaking into NH3 and H+ ions, and thus not all NH3 results in the formation of OH- ions, and thus NH3 is a weak base.
Some chemical/physical properties of ammonia are:

- Ammonia is a colorless, very unpleasant gas with a terrible, stifling stench at room temperature.
- It is hygroscopic and is there as anhydrous ammonia in its pure state (readily absorbs moisture).
- Ammonia is corrosive and has alkaline characteristics.
- Ammonium hydroxide, an acidic solution, and weak Base form when ammonia gas dissolves easily in water.
- Under pressure, ammonia gas compresses easily and becomes a transparent liquid.
- Ammonia is frequently getting transported in steel containers as a compressed liquid.
- Although ammonia is not very flammable, it can explode when subjected to tremendous heat.