Diffusion is the final movement of anything normally from a part of higher concentration to a part of lower concentration. As a result, the idea of diffusion is widely used in many areas, including physics (particle diffusion), chemistry, biology, economics, sociology, and finance (diffusion of people, and price values). The term “diffusion” comes from the Latin word “diffundere”. It means “to spread out.” However, the main idea of it is common to all of these. Likewise, a material or collection going through diffusion opens out from a point or area at which there is a higher mixture of that material or collection.

Meaning of Diffusion
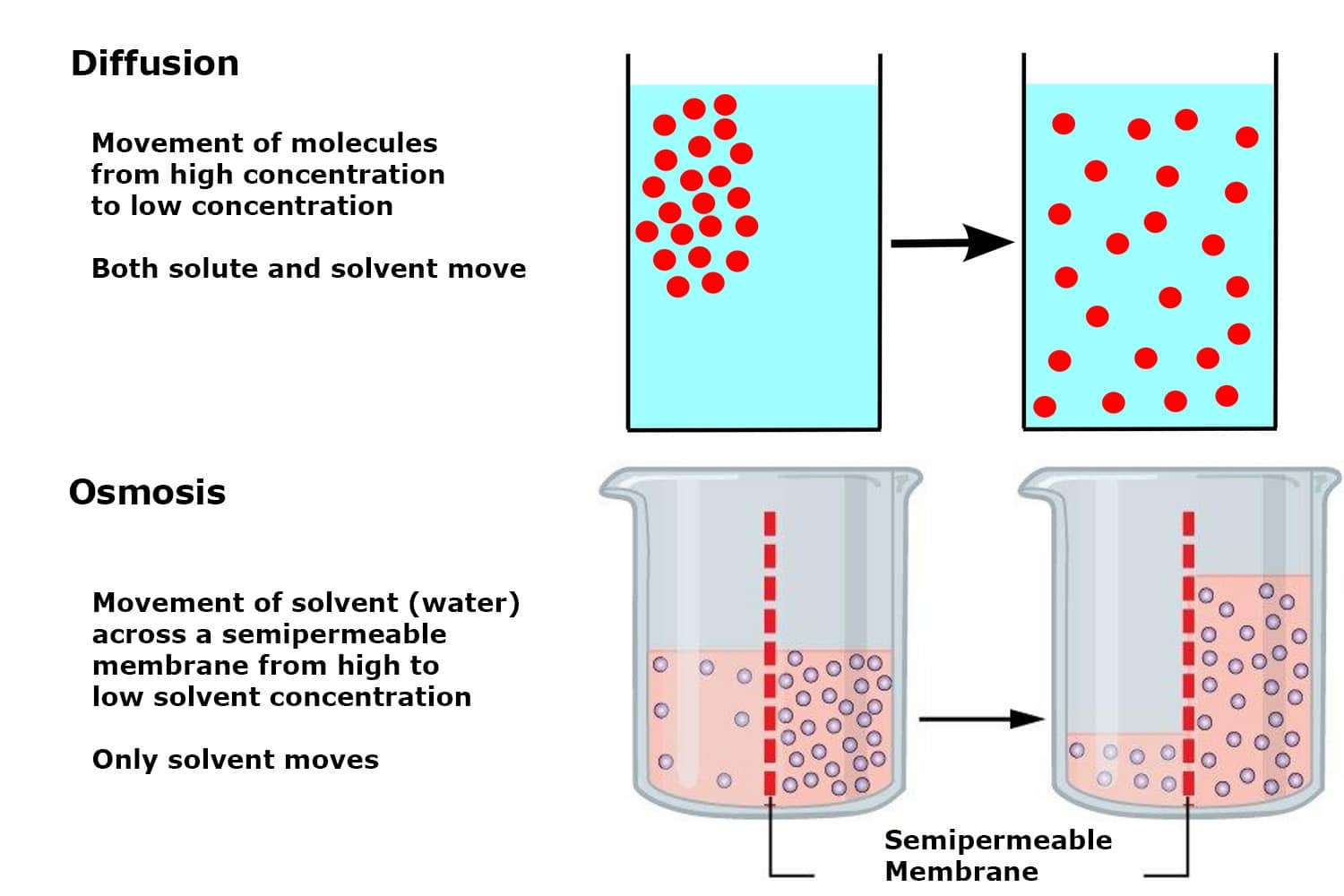
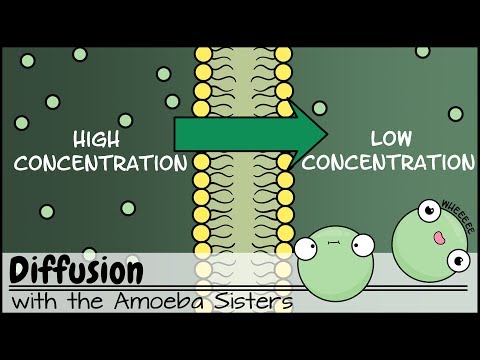
Diffusion is the process of the flow of particles under a concentration gradient. The gradient is a slope, which may be upward or downward. It is an important process taking place in all living beings. It helps in the moving of particles in and out of the cells. The particles move from a place of higher potential to a place of lower potential until the concentration becomes equal throughout.
Liquid and gases spread as the particles are able to move freely.
Diffusion in Physics
The moving of particles from an area of higher potential to lower potential as moved by heat energy is diffusion. For example, the particles put up in liquids and gases, hit one another resulting in their free never-ending motion. This motion is due to the hitting of particles known as the Brownian movement. These particles increase in number. As a result, they become settled and the Brownian movement is clearly lost. We can see this in a concentrated solution. When we give it with an increased space they start to move. However, they follow Fick’s laws moving in an order from an area of high concentration to low concentration.
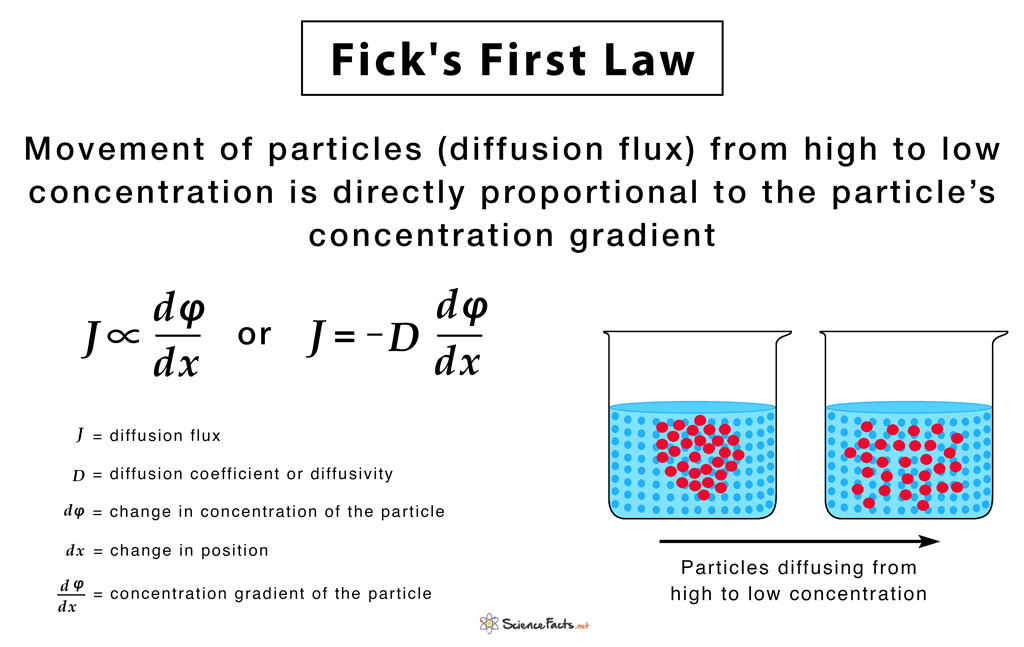
Temperature, concentration, distance, and material affect the spreading rates. In particular, a hot temperature begins the particles to gain more kinetic energy. Kinetic Energy is the energy owned by the particles in motion. For this reason, it will move and push other particles more. And so, the higher the temperature, the faster the spreading of particles.
On the other hand, it is slower at a lower temperature. The greater the number of particles in a solution, the faster is the rate of spreading for particle concentration. The shorter the distance for the particles to travel, the faster is the rate of diffusion, as for the distance. The rate of diffusion is also affected by the kind of material in the solution. Smaller and lighter particles can roll out more easily than larger and heavier ones. As a result, gaseous particles spreads faster than the particles in liquids or solids. In the same way, liquids spread faster than solids.
Diffusion in Chemistry
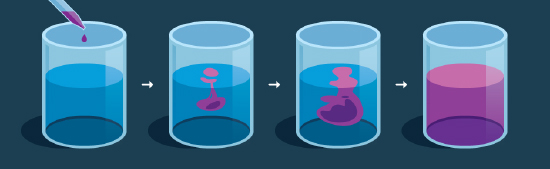
The movement of a liquid from an area of higher concentration to a lower concentration is known as diffusion. It is a result of the moving effect of particles of matter. The particles will mix till the time they are equally distributed.
For example, food colouring in water spreads until it’s equally spread completely in the liquid.
In diffusion, particles move down a collection slope. It is different from other transfer processes. As a result, it mixes without major matter flow. For this reason, those molecules in motion from heat energy move for free. In the end, this free motion leads to an even distribution of different particles. It is true, atoms and particles only seem to move freely. Most of their motion results from hitting other particles. Rising temperature or pressure increases the rate of spreading.
Diffusion in Biology
A physical process referring to the total motion of particles from a place of higher potential to one of lower potential is diffusion. The movement stops when the concentration of the substance is equal in both places. This does not mean that the particles of a substance are not moving anymore. In short, there is no motion in one direction at all. Atoms of a substance move equally in both ways. In biology, it is a passive transport method. Passive transport is a way where small particles move across the cell membrane without the input of energy by the cell.
A layer that is selectively porous has control over what atoms or ions can enter or leave the cell. The ability of a membrane to pass is depending on the shaping and features of the layer lipids and proteins. As a result, cell membranes help to keep a state of homeostasis within cells (and tissues, organs, and organ systems). For this reason, a life form can stay alive and active. A selectively passable layer allows some atoms through, but not others.
There are two other ways that particles can pass through a phospholipid layer. In the first place, Active transport needs that the cell uses energy to pull in or give out some molecules and ions. Furthermore, the second way is through vesicle movement, where large particles are moved across the layer in bubble-like packs made from pieces of that layer.
Diffusion in Plants and Animals
Plants also need food, water, and minerals for their growth and survival like every other living thing. The root of the plants helps in taking in water and minerals from the soil. Leaves use this to make their food. Then, the prepared food is moved to the other parts of the plants. Here, we are talking about moving the food. As a result, there must be some way of transportation. Therefore, diffusion is the process of movement followed by plants.
:max_bytes(150000):strip_icc()/photosynthesis_gas-0f67c6be034c4e81ae55e2e5a7a30d1f.jpg)
Our body is made up of cells, which is referred to as the growing as well as a working unit of life. The cells present in our body need glucose and oxygen (O2) for breathing. Both these parts are carried in the blood. As a result, when blood carrying these elements reaches the cells, the particles of glucose and oxygen divide out of the blood and into the cells. After using this glucose and oxygen, cells make waste chemicals at the same time with carbon dioxide gas (CO2). The presence of these waste chemicals and carbon dioxide gas would poison the cell. For this reason, they spread out of the cells into the blood.
Diffusion in Cells
The cell controls the entry and exit of elements through its plasma skin. This layer has been planned to allow certain molecules to pass while others cannot. It is the lipid bilayer that blocks the passing of polar molecules through the plasma skin. However, it is possible for nonpolar molecules and ions to pass through the lipid bilayer.
Types of Diffusion
There are three types of diffusion. They are as follows:
- Simple diffusion,
- Osmosis,
- Facilitated diffusion.
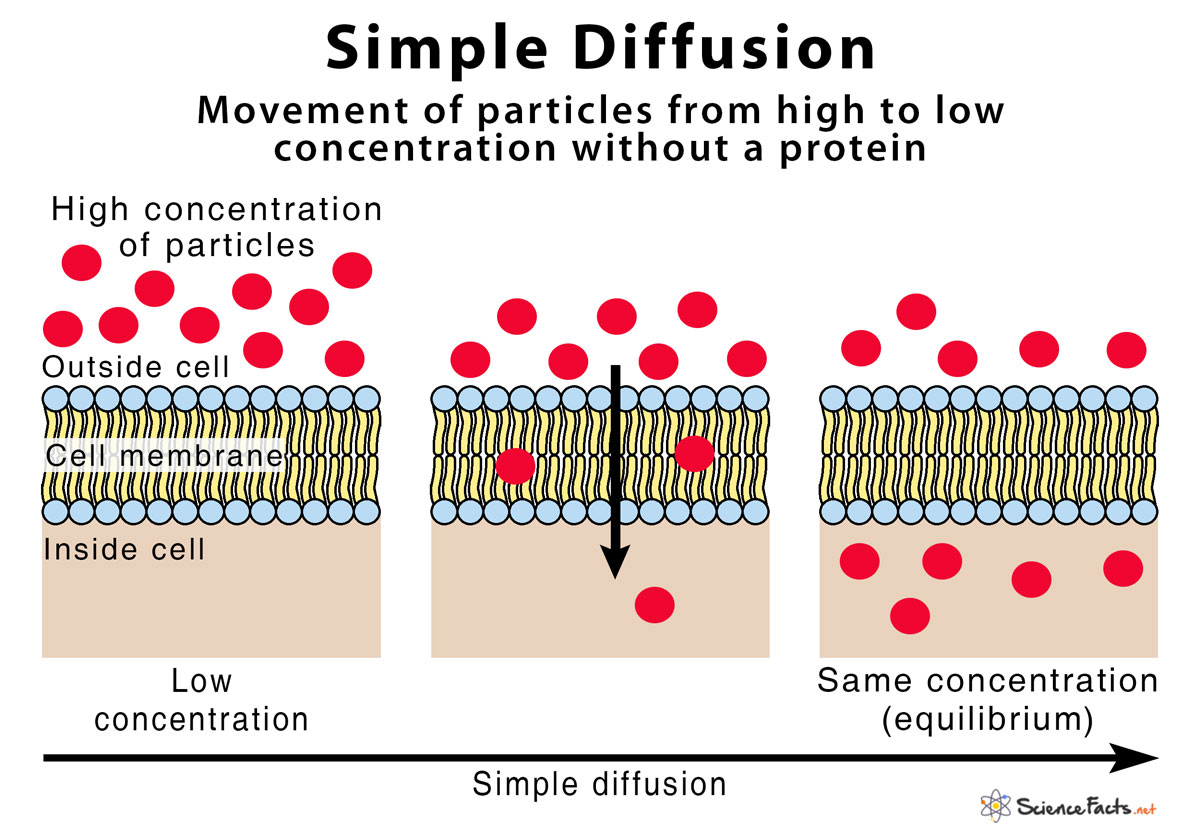
Simple Diffusion
This is a form of diffusion that does not need the help of membrane proteins. Chiefly, the particle moves from higher to lower potential. However, its motion does not need a membrane protein that will help substances to move down. In physics and chemistry, diffusion is explained mainly as the “spreading out” of the objects from the first area of higher concentration. However, in a biological system, the same idea applies but the process needs a semipermeable biological layer.
ATP (a chemical form of energy) does not directly push simple diffusion. In the same way, as in other processes, the energy that powers simple diffusion is kinetic energy and concentration gradient. The molecules strike each other. As a result, particles are in free continuous motion. The striking of particles is called pedesis. The particles are more likely to be crowded when an area is filled. This reduces the motion as well. For this reason, when a larger space becomes free, the particles likely move towards an area with a larger space.
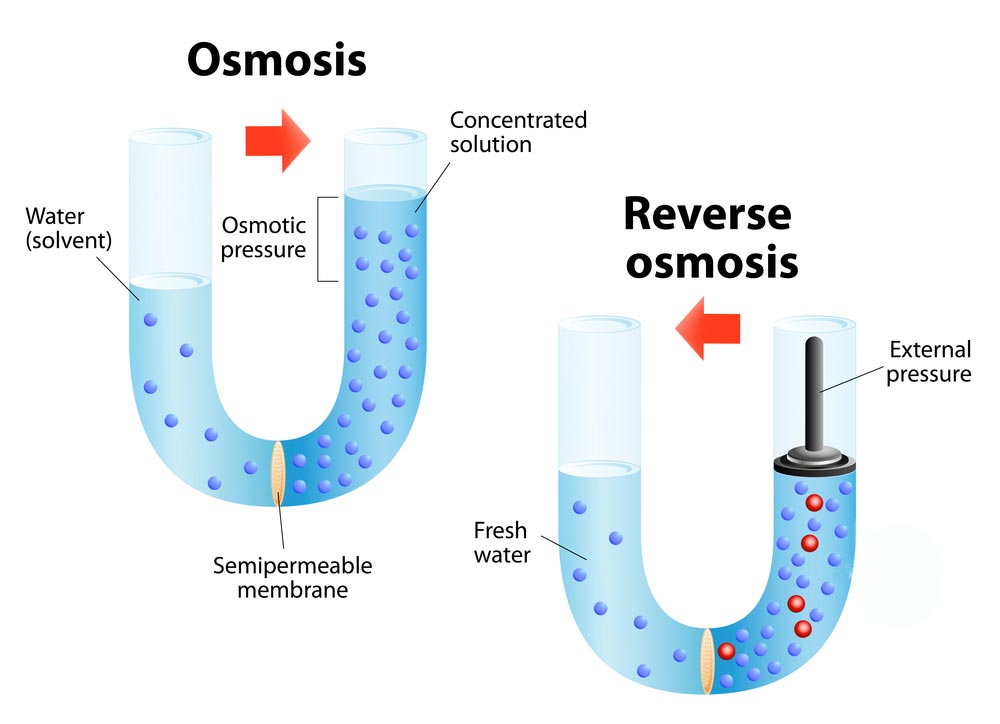
Osmosis
Osmosis is a passive process taking place without any wastage of energy. It contains the movement of molecules from a region of higher concentration to lower concentration. It takes place until the concentrations become equal on either side of the membrane. Any solution can go through the process of osmosis including gases and liquids.
Osmosis affects the cells in different ways. An animal cell will dissolve when placed in a hypotonic solution. On the other hand, a plant cell probably would not. The plant cell has thick walls and needs more water. The cells will not crack when placed in the solution. In fact, a hypotonic solution is perfect for a plant cell.
An animal cell survives only in an isotonic solution. In an isotonic solution, the plant cells are no longer puffy and the leaves of the plant drop. We can stop or change the osmotic flow. It is Reverse osmosis. We do it by applying outer pressure to the sides of the solute. The minimum pressure needed to stop the solvent transfer is the osmotic pressure.
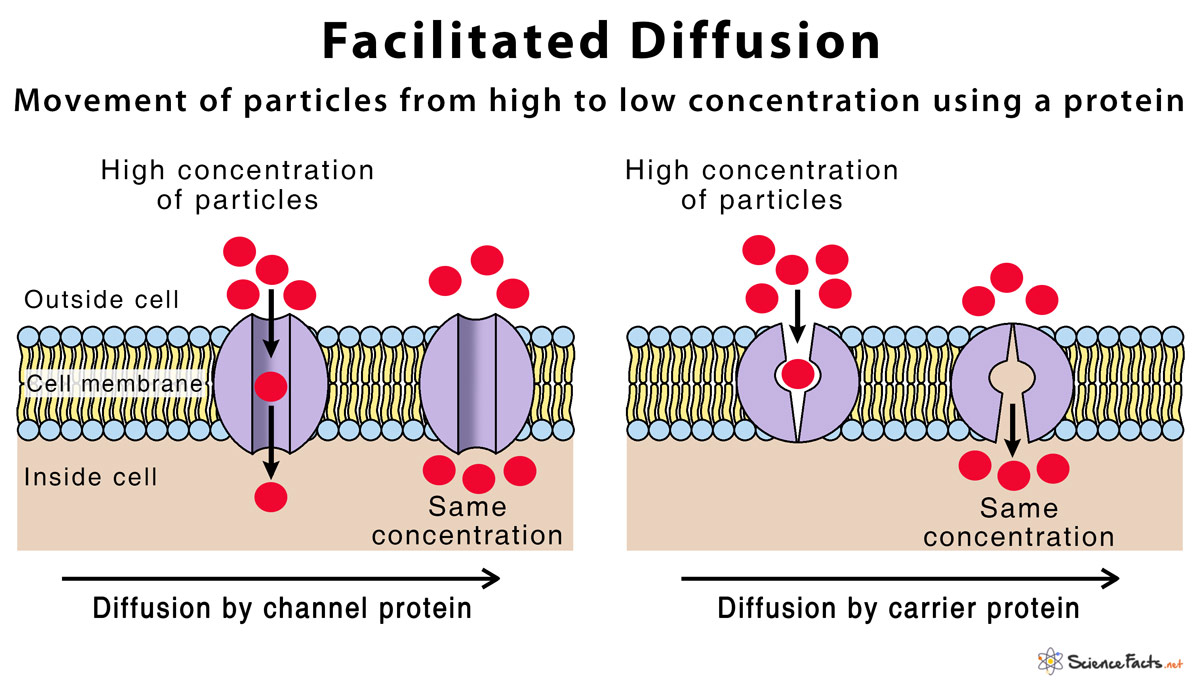
Facilitated diffusion
Facilitated diffusion is the moving of substances over a biological layer from an area of higher potential to lower potential with the help of a moving particle. Chemical energy is not directly needed to move substances in the direction of their concentration gradient. Examples of processes that need facilitated diffusion are glucose and amino acid transport, gas transport, and ion transport. This is major because it controls what goes in and what goes out of the cell. The plasma layer is the cellular form in charge of the selective motions of particles.
Diffusion and Osmosis
Here, are some differences between diffusion and osmosis below:
- Osmosis is mainly the moving of water from higher to lower potential. Whereas, diffusion takes place in liquid, gas and even solids.
- Osmosis needs water for the moving of particles. Whereas, it does not need water for the moving of particles.
- The flow of particles takes place only in one direction in osmosis. But, the flow of particles takes place in all the directions
- Osmosis takes place only between the same types of solutions. On the other hand, it takes place between the same and different types of solutions.

Factors that affect Diffusion
- Concentration- If the difference in concentration is higher, the particles move towards the area of lower concentration faster.
- Temperature- The particles move faster at higher temperatures. Whereas, they slow down at lower temperatures.
- Mass of Particle- Heavier particles move more slowly resulting in a slower rate of diffusion. On the other hand, smaller particles spread faster because they can move faster.
- Solvent Properties.
Examples of Diffusion
- A tea bag placed in a cup of hot water will spread into the water.
- The smoke rolls out to all parts of a room after lighting a cigarette.
- Water spreads into cooking noodles making them bigger and softer.
- Sugar melts evenly and sweetens the water without having to mix it.
Importance of Diffusion
- Change of gases through stomata openings is possible because of this.
- Transpiration is the loss of the extra amount of water that works on the idea of diffusion.
- The moving of prepared food to different parts.
- The moving of food material.
- It is important to cells because it allows them to gain useful elements. We need it to get energy and grow. It helps us to get rid of waste products.
FAQs (Frequently Asked Questions)
1. What is the significance of diffusion?
It is a very important process taking place in all living beings. All living organisms show one or the other form of diffusion. This allows the moving of the molecules during many living processes.
2. Is osmosis a type of diffusion?
Yes.
3. What is passive transport?
It is a type of transport that does not need any energy to move substances between cells.
4. How is diffusion different from Osmosis?
It takes place in the liquid form only. It needs water for the moving of particles. Whereas, diffusion takes place in liquid, gas and even solids. It does not need water for the moving of particles.
5. What is Active Transport?
It is the process of moving molecules across a living layer through the use of cellular energy.
6. What are the types of diffusion?
Simple diffusion, Facilitated diffusion and Osmosis.
7. How does it happen in cells?
Particles can move into and out of cells through the cell membrane.